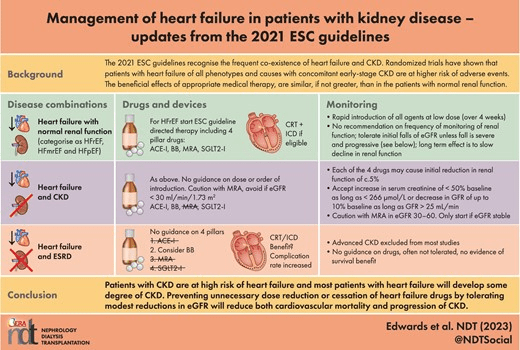
Management of heart failure in patients with kidney disease—updates from the 2021 ESC guidelines
by Nicola C Edwards, et al.
Nephrology Dialysis Transplantation, Volume 38, Issue 8, August 2023, Pages 1798–1806, https://doi.org/10.1093/ndt/gfad011
The 2021 guidelines address the co-existence of HF and CKD in a short section titled ‘Non-cardiovascular co-morbidities’. This section remains brief as in 2016 and exemplifies the difficulty in extrapolating prospective HF RCT data when advanced stage CKD (eGFR <20–30 mL/min/1.73 m2) remains an exclusion criterion in most studies. The authors do emphasize that randomized trials have shown that patients with HF of all phenotypes and causes with concomitant early-stage CKD are at higher risk of events and that the beneficial effects of appropriate medical therapy, are similar, if not greater, than in the patients with normal renal function. They describe the significant benefits of using ACE-I/ARNI and BB in moderate CKD (eGFR >30 mL/min/1.73 m2) based on data from landmark trials compared with subjects with normal renal function. Data from sub-group analyses have highlighted the absence of interaction between drug benefits and renal function. We would also highlight the consistent and accumulating evidence from SGLT2-I trial literature for the benefits of reducing the progression of renal disease irrespective of HF or existing atherosclerotic cardiovascular disease (Fig. 3). Indeed, the recently published EMPA-KIDNEY trial demonstrated that empagliflozin is both safe and efficacious in reducing the risk of kidney disease progression or death from a cardiovascular cause in a cohort with a wide range of renal function (eGFR 20–90 mL/min/1.73 m2) and proteinuria [22]. Proportional benefits were observed across a range of renal diagnoses and with a less than a third of patients having known cardiovascular disease.
<img src="https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/38/8/10.1093_ndt_gfad011/1/m_gfad011fig3.jpeg?Expires=1712997789&Signature=uW-w8r4yIx0YEbH5EKK6gOVB1dNKU73PVwhEUdlnoPZxSl07n86BhjGcWxFu7PHURj0tyARAkOCCeW2Rq0y4ozUKwUD1CbkQZ1UADhvLSZkNcNN9sVRrXDY8NW13TG5W5p7ITZY5LToW2ES8kEFvZU-4X0oxIISUc81y45LZ7ABFnPpVrX6erRZZgPp3dabbGgzdn0CScsn~RxWeGRC3d7Ved1CGMInAgqRniEgA8OWHeVmHXHgAi3bvfXaH4gyRbuG9jsVF2zGTDih-wzVoim6GMi57jUq0UpJ~s5YNhHmRTQEZEkIlXmfHlhOraFDvqBlu59eg2dVE7I9Go-ZWNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA" alt="The overlap between SGLT2-I RCTs in those with CKD and HF. HF and CKD are closely associated and share common comorbidities and traditional risk factors. The renal and cardiovascular endpoints in this diagram are taken from four recent trials; two which recruited patients with CKD (CREDENCE and DAPA-CKD) and two which recruited patients with HF (DAPA-HF and EMPEROR-Preserved). Beneficial effects of treatment with SGLT2-I were seen on both renal and cardiovascular endpoints in both groups of trials. *The EMPEROR-Preserved trial excluded patients with a GFR <20 mL/min/1.73 m2 and the DAPA-HF trial excluded participants with a GFR
Figure 3: The overlap between SGLT2-I RCTs in those with CKD and HF. HF and CKD are closely associated and share common comorbidities and traditional risk factors. The renal and cardiovascular endpoints in this diagram are taken from four recent trials; two which recruited patients with CKD (CREDENCE and DAPA-CKD) and two which recruited patients with HF (DAPA-HF and EMPEROR-Preserved). Beneficial effects of treatment with SGLT2-I were seen on both renal and cardiovascular endpoints in both groups of trials. *The EMPEROR-Preserved trial excluded patients with a GFR <20 mL/min/1.73 m2 and the DAPA-HF trial excluded participants with a GFR <30 mL/min/1.73 m2. CV: cardiovascular; RRT: renal replacement therapy.
The guidelines do not provide specific guidance on the use of the four pillar therapies in patients with pre-existing CKD including order of introduction, dose adjustment or frequency of monitoring of kidney function, but independent groups have proposed strategies according to eGFR [16]. Given the clear evidence of benefit, the use of the four pillar drugs and their rapid introduction in HFrEF with non-dialysis CKD should be strongly supported by nephrologists allowing for usual cautions and close monitoring of serum potassium. Indeed, withholding this new treatment approach would seem only to perpetuate the often cited complaint of therapeutic nihilism in patients with CKD. More recent evidence on drugs such as finerenone (a non-steroidal MRA) and SGLT2-I provides reasons for optimism that the very high adverse cardiovascular event rate in CKD can be effectively reduced (Fig. 4). In DAPA-Kidney, the beneficial effects of dapagliflozin on cardiovascular death and HHF in advanced CKD (eGFR 25–30 mL/min/1.73 m2) were comparable to that seen in HFrEF with early-stage CKD [50]. Finerenone effectively reduced the same endpoints in patients with diabetic CKD [51]. Two reviews summarize these data well [52, 53].

Figure 4: Timeline of randomized controlled drug trials in those with CKD with cardiovascular mortality or morbidity endpoints. This diagram details the progress made in our knowledge of reducing cardiovascular morbidity and mortality in patients with CKD. Studies in the purple box took place prior to 2014. Studies in the yellow box are currently registered as ongoing clinical trials with ClinicalTrials.gov and results are expected this year. ALCHEMIST: Aldosterone antagonist chronic haemodialysis interventional survival trial; AURORA: A study to evaluate the use of Rosuvastatin in subjects on regular haemodialysis: CANVAS: Canagliflozin and renal events in diabetes with established nephropathy clinical evaluation; CREDENCE: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy; DAPA-CKD: Dapagliflozin and prevention of adverse outcomes in chronic kidney disease; DAPA-HF: Dapagliflozin and prevention of adverse outcomes in HF; DECLARE-TIMI: Dapagliflozin effect on cardiovascular events-thombolysis in myocardial infarction; DOHAS: Dialysis outcomes HF Aldactone study: DCOR: The dialysis clinical outcomes revisited; EMPA KIDNEY: The study of heart and kidney protection with empagliflozin; EMPA-REG: Empagliflozin cardiovascular outcome event trial in type 2 diabetes mellitus patients; EMPEROR-Preserved: Empagliflozin outcome trial in patients with chronic HF with preserved ejection fraction; EMPEROR-Reduced: Empagliflozin outcome trial in patients with chronic HF with reduced ejection fraction; FIDELIO-DKD: The Finerenone in reducing kidney failure and disease progression in diabetic kidney disease; FIND CKD: A trial to learn how well finerenone works and how safe it is in adult participants with non-diabetic chronic kidney disease; FIGARO: Cardiovascular events with finerenone in kidney disease and type 2 diabetes; SHARP: Study of heart and renal protection; 4D STUDY: Die Deutshe diabetes dialyse studies; PIVITOL: Proactive Intravenous Iron Therapy in Haemodialysis Patients; TREAT: Trial to reduce cardiovascular events with Aranesp therapy; VERTIS CV: Evaluation of ertugliflozin efficacy and safety cardiovascular outcomes trial.
Historically, renal medicine, unlike cardiology, has had a poor track record for producing good quality, large-scale RCTs [54]. Furthermore, patients with CKD stage 4 or higher are routinely excluded from cardiovascular trials [54]. Pressure should continue to be put on regulatory authorities demanding the inclusion of patients with CKD of all stages in trials by governments and learned societies such as the European Renal Association (ERA). Similar approaches together with incentivizing measures have already improved the recruitment of women, children, elderly people and ethnic minorities into RCTs.
The evidence-based renal landscape is changing rapidly. Even while writing this review, a number of pivotal and practice changing RCTs in both CKD and HF have been published utilizing SGLT2-I, non-steroidal MRA and iron, which do not feature in the published guidelines but would strongly support their use in CKD-HF phenotypes. The next challenge is to produce rapid communication channels which translate the results of these studies into more timely guidance for practising clinicians. Guidelines from expert societies take a long time to produce and can often end up disagreeing with each other, leading to confusion [55]. A collaborative approach from working groups of different societies such as the ESC and ERA would lead to guidance on ‘crossover’ clinical issues including HF and CKD that can be produced much more quickly and updated rapidly as evidence emerges.
